The lack of high-resolution imaging tools to monitor disease progression is one of the key hurdles in understanding the pathophysiology of intervertebral disc (IVD) degeneration in preclinical models. Although magnetic resonance imaging (MRI) is commonly used to quantify IVD morphological and compositional changes in vivo for humans and large animal models, the MRI has limited utility in rodent models of degeneration.

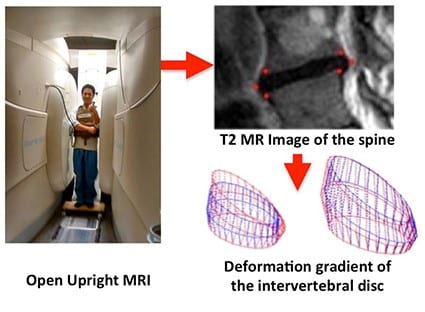
We have developed contrast-enhanced micro-computed tomography (microCT) techniques for the high-resolution imaging of the rodent IVD in vivo. This method of imaging improves the resolution of the state-of-the-art microMRI by approximately 5,000 to 100,000 fold, and it has enabled us to longitudinally monitor the injury and degenerative cascade of the IVD in our mouse models and discern the effectiveness of our therapies. This is a key technology innovation in our NIH R21AR069804/R01AR074441 studies.At the clinical scale, we have optimized and adapted the use of Open Upright MRI system for patient-specific imaging combined with finite element analyses. While most clinical MRIs in the supine position fail to correlate with the clinical presentation of non-specific low back pain, we have developed a clinical workflow that enables imaging in a loaded positions to provide additional context on the function of the IVD during activities of daily living. As the spine undergoes physiological postures, it experiences a wide range of loads, and the IVD transmits and responds to the loads through its deformations and subsequent strains at the tissue level. Evaluating these strains can directly account for the mechanical function of the IVD, and these strains may be relevant to pain presentation in individuals whose LBP is provoked by specific physiological postures. We are calibrating this approach in human participants with inducible low back pain, we have been successful in identifying the spine regions that are most susceptible to pain and damage. This approach has the potential to significantly improve the diagnostic specificity of painful spinal pathologies. This is the key technology innovation in our NIH K01AR069116 study.
Observing the initiating mechanisms of IVD degeneration is difficult and costly in humans, particularly when there are many confounding and complex factors that contribute to IVD degeneration and eventual LBP. In a laboratory setting however, the organ culture approach enables mechanistic modulation of homeostatic variables by reducing the experimental system to only the native cell population and their surrounding extracellular matrix and microstructures. Such systems make it easier to interpret the direct effects of external stimuli, how they may lead to IVD degeneration, and their ultimate functional and mechanical consequences. Murine models provide an opportunity to rapidly achieve IVD homeostasis, apply experimental stressors, and leverage the use of the well-established assays and genetically engineered animals to better understand the mechanisms that drive degeneration.
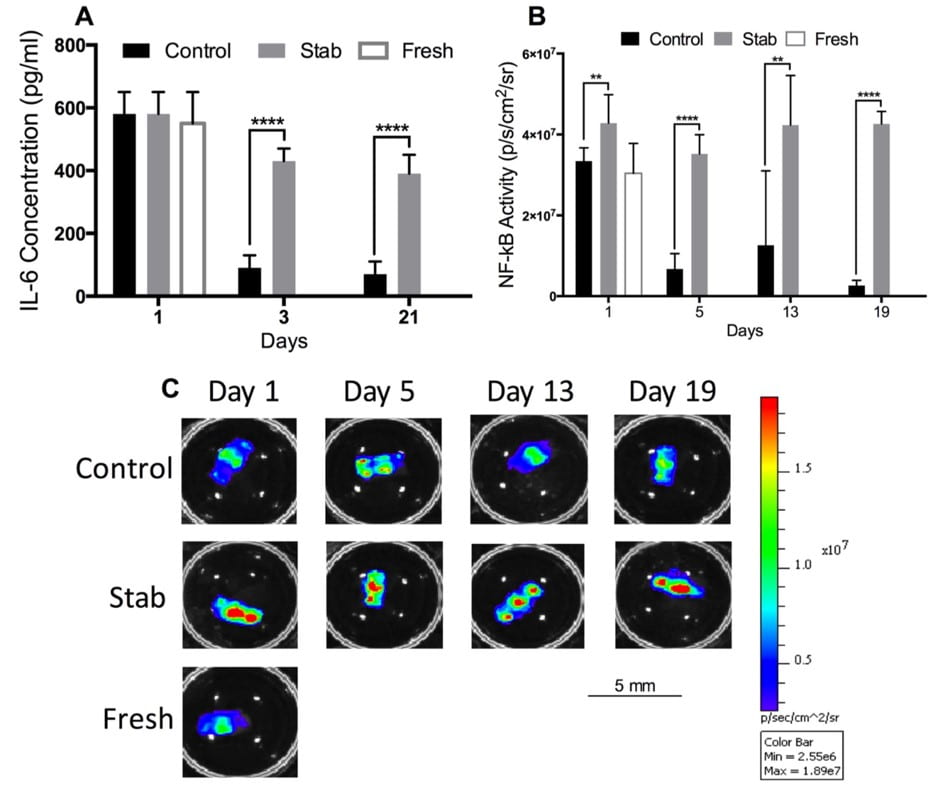
We have previously developed and validated an in vitro organ culture system for mouse lumbar functional spine units (FSUs). Utilizing the whole FSUs maintains both the bony and cartilage endplates which have been shown to be critical for nutrient diffusion into the IVD and function. Using this approach, we have shown the intervertebral disc of a mouse lumbar FSU can be maintained with sufficient nutrition and viable up to at least 21 days. Moreover, we successfully monitored the sustained inflammatory response of mechanically induced injury that caused progressive degeneration. This approach offers several unique experimental advantages: (1) multiple FSUs can be obtained from the same animal enabling a more robust experimental design; (2) experimental consistency through culturing multiple FSUs on a standard culture plate to homogenize the exposure conditions of each sample; (3) multiple factors can be monitored robust cytokine production can be monitored 2-3 days during the culture period from the exchanged media; (4) the IVD remains responsive to stimuli by mechanical injury or by molecular inflammatory perturbations (e.g. by administration of LPS – Lipopolysaccharides); and (5) utilization of genetic modified animals with molecular reporters enable further real-time monitoring of the FSU tissue. An example of the multiplexed measurements is shown in Figure 1. Taken together, this platform enables the high-throughput and repeatable screening of novel drugs and therapies.
Advanced glycation end-products (AGEs) are non-enzymatic sugar-based modifications of collagen that accumulate with aging, diabetes, and drug treatment, and increased AGEs impair the material properties of the extracellular matrix and contribute to skeletal fragility. Although the role of AGEs in bone has been studied extensively, much less is known about their role in the IVD. AGEs accumulates in the IVD with aging and progressive degeneration, and the increased AGEs may adversely affect the material properties, hydration status, and structural biomechanics of the IVD. Beyond the biomechanical effects, AGEs may play a signaling role in the inflammatory cascade that leads to abnormal matrix synthesis and early apoptosis in disc cells, consistent with observations in the degenerating disc. The receptor-of-advanced-glycation-endproducts (r-AGEs) may be a potential mechanism that initiates the cellular degeneration of the IVD, leading to pain and matrix destruction. We have demonstrated a causative role between advanced glycation end-products, r-AGEs, and inflammatory cytokines as well as other deteriorations in IVD structure-function. We are examining biologics and other targets for r-AGEs mediated inflammatory behavior as well as rescuing the effects of NEG-mediated mechanical changes in the disc. This work is supported by NIH R21AR069804/R01AR074441.
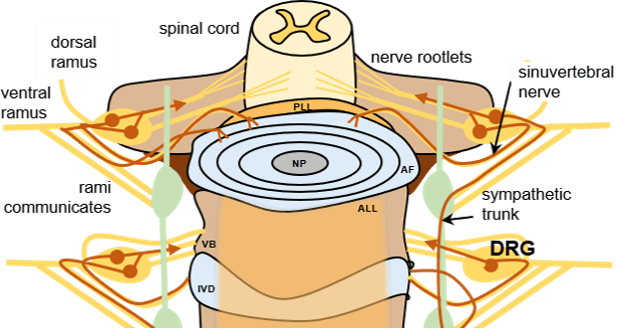
IVD degeneration contributes to chronic low back pain in up to 40% of cases, with an annual burden of over $100 billion in healthcare and related costs. Nevertheless, a role for degenerated IVDs as pain generators remains poorly understood. To date, our understanding of “cross-talk” between the nervous system and the degenerated IVD has relied upon observations of changes in molecule and RNA expression for key cytokines, neurotrophins, ion channels and cell markers in IVD tissues, IVD conditioned media or dorsal root ganglia (DRG) neurons. Pre-clinical models of IVD injury, together with imaging of sensory neuron activation, provide an opportunity to study the temporal and spatial “cross-talk” between neuronal function and the IVD following injury. In preliminary work, we showed that lumbar disc degeneration (L5-L6) in the rat following was associated with changes in ion channel activation in L1 and L2 DRG neurons at 20 weeks after surgery to induce IVD degeneration; these changes involved impaired generation of Ca2+ transients as well as protein expression of the heat-sensitive TRPV1 ion channel in DRG neurons. We propose to evaluate the temporal onset of action potential-driven Ca2+ transients and molecular changes for multiple ion channels in sensory neurons of a mouse IVD injury model. In addition to studying the onset of IVD injury and associated changes to mouse behavior and sensitivity, we will test if, where and when ion channel function is modified in DRGs in vivo and in vitro. This work will illustrate the molecular and functional changes in nervous system-IVD “cross-talk” associated with a transition from acute to chronic pain in IVD injury and degeneration.
We are also investigating the role of growth factors that promote innervation in the IVD. The proliferation of sensory nerves is tightly regulated by Nerve Growth Factor (NGF) and Vascular Endothelial Growth A (VEGFA). NGF serves critical homeostatic and survival functions in neurons, while VEGFA, in addition to initiating angiogenesis, plays a crucial role in spontaneous neurite extension and dendritic outgrowth in connective tissues. Degenerate and injured IVD cells express VEGFA as a part of the inflammatory cascade, and anti-VEGF therapies have shown promise in reducing downstream TNFa activity within the IVD and decreasing activation of neighboring dorsal root ganglions. However, whether VEGFA plays a role in the neoinnervation observed in the degenerate IVDs of rodents exhibiting chronic low back pain is unknown.
We are also currently exploring receptors responsible for damage sensing and inflammatory control of the IVD’s extracellular environment. These works represent fundamental mechanobiology of the IVD with the potential to become therapeutic targets for the inhibition of IVD injury, degeneration, and subsequent pain.
IVD degeneration contributes to chronic low back pain in up to 40% of cases, with an annual burden of over $100 billion in healthcare and related costs. Nevertheless, a role for degenerated IVDs as pain generators remains poorly understood. To date, our understanding of “cross-talk” between the nervous system and the degenerated IVD has relied upon observations of changes in molecule and RNA expression for key cytokines, neurotrophins, ion channels and cell markers in IVD tissues, IVD conditioned media or dorsal root ganglia (DRG) neurons. Pre-clinical models of IVD injury, together with imaging of sensory neuron activation, provide an opportunity to study the temporal and spatial “cross-talk” between neuronal function and the IVD following injury. In preliminary work, we showed that lumbar disc degeneration (L5-L6) in the rat following was associated with changes in ion channel activation in L1 and L2 DRG neurons at 20 weeks after surgery to induce IVD degeneration; these changes involved impaired generation of Ca2+ transients as well as protein expression of the heat-sensitive TRPV1 ion channel in DRG neurons. We propose to evaluate the temporal onset of action potential-driven Ca2+ transients and molecular changes for multiple ion channels in sensory neurons of a mouse IVD injury model. In addition to studying the onset of IVD injury and associated changes to mouse behavior and sensitivity, we will test if, where and when ion channel function is modified in DRGs in vivo and in vitro. This work will illustrate the molecular and functional changes in nervous system-IVD “cross-talk” associated with a transition from acute to chronic pain in IVD injury and degeneration.
We are also investigating the role of growth factors that promote innervation in the IVD. The proliferation of sensory nerves is tightly regulated by Nerve Growth Factor (NGF) and Vascular Endothelial Growth A (VEGFA). NGF serves critical homeostatic and survival functions in neurons, while VEGFA, in addition to initiating angiogenesis, plays a crucial role in spontaneous neurite extension and dendritic outgrowth in connective tissues. Degenerate and injured IVD cells express VEGFA as a part of the inflammatory cascade, and anti-VEGF therapies have shown promise in reducing downstream TNFa activity within the IVD and decreasing activation of neighboring dorsal root ganglions. However, whether VEGFA plays a role in the neoinnervation observed in the degenerate IVDs of rodents exhibiting chronic low back pain is unknown.
We are also currently exploring receptors responsible for damage sensing and inflammatory control of the IVD’s extracellular environment. These works represent fundamental mechanobiology of the IVD with the potential to become therapeutic targets for the inhibition of IVD injury, degeneration, and subsequent pain.
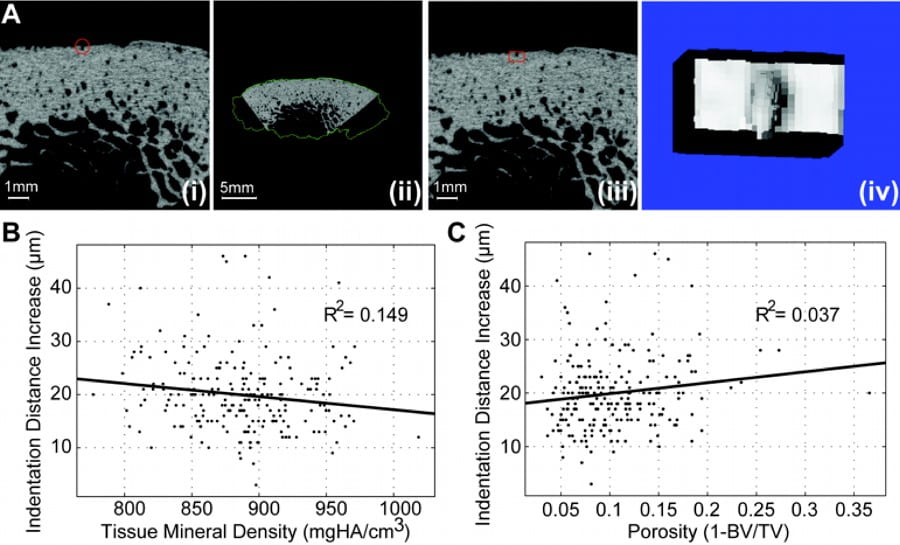
DXA (Dual energy X-ray Absorptiometry), the clinical standard for bone fracture risk, while valuable and informative, is substantially limited in the prediction fracture and monitor drug therapies. In addition to the investigation of fracture mechanisms, we have also developed patient-specific methods to assess fracture risk. The fracture resistance of bone is ultimately determined by bone’s ability to effectively dissipate mechanical energy, and mechanical testing is the most direct way to assess fracture resistance. Because the obvious destructive nature of traditional mechanical testing, as well as the scarcity of tissues and the invasiveness of biopsies to the patient, mechanical testing of bone tissue to determine its competence has never before been feasible in a clinical setting. In collaboration with industry partners, we will further investigate the predictive nature of minimally invasive microindentation towards whole bone fragility and stress fractures. This project will develop and extend measures of bone quality in the clinical assessment of fracture risk in conjunction with existing technologies. The results would be particularly applicable to age-related deterioration of bone quality, as well as the in vivo monitoring of alterations due to pharmaceutical treatments and therapies. This was supported by NIH R43-AG060607.