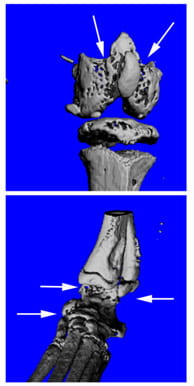
During rheumatoid arthritis (RA), increased immune cell activation leads to focal bone erosion as well as systemic bone loss, both mediated by osteoclasts (OCs). Dendritic cells (DCs) are critical components of the inflammatory response associated with RA as they are responsible for the activation of T lymphocytes, due to abnormal presentation of self-antigens. Neutrophils can further enhance the inflammatory condition by recruiting more DCs and contribute to local bone erosion by actively secreting inflammatory and pro-osteoclastogenic cytokines. Unfortunately, traditional pharmacotherapy to block the activity of inflammatory cytokines can be inadequate in controlling symptoms and disease progression. Our overall goal is to identify signaling molecules that are critical to modulate the inflammatory and osteolytic components of RA and that may lay the groundwork for novel and more effective therapeutic approaches. We have previously found that PLCγ2 is a critical modulator of bone homeostasis and inflammatory responses associated with RA. However, PLCγ2 shares high homology with the more ubiquitously expressed PLCγ1, thus rendering its specific targeting difficult. Diacylglycerol (DAG) is a downstream product of PLCγ2 catalytic activity and data from our group and others indicate that it mediates PLCγ2 function in OCs and immune cells. In order to study the role of DAG, we established animal models of DAG overexpression or deletion of specific DAG-dependent downstream effectors. The goal of our studies is to demonstrate the importance of DAG-dependent pathways in the activation and functionality of OCs, neutrophils and DCs as a way to target both the osteolytic and inflammatory components of RA. This novel approach might lay the basis for new therapeutic interventions for RA.
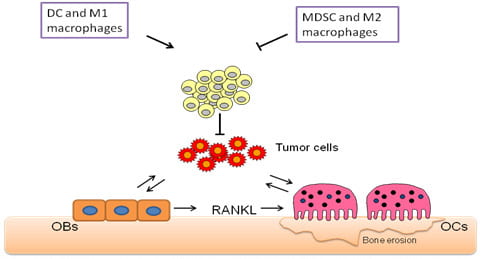
Breast cancer remains one of the major killers of women in this country and bone metastases represent one of the most serious complications of the disease. Bone metastases are found in about 80% of breast cancer patients and are associated with increased morbidity as reflected through pain, bone fractures and reduced quality of life. The metastasized cells disrupt normal bone remodeling pathways resulting in increased number of osteoclasts (OCs) and release of some bone stored growth factors that further promote tumor growth. Thus, a cycle of metastatic bone destruction, knows as tumor/bone vicious cycle, is initiated, leading to increase osteoclastic response and compromised skeletal integrity. Animal studies using human breast cancer cells injected in immune compromised animals have demonstrated that blockade of OC function reduces bone tumor growth and prevents associated bone loss. Hence the bone resorptive polykaryon is a principal therapeutic target in afflicted individuals. Clinical studies indicate that inhibition of OC activity is effective in reducing skeletal complications such as bone pain, pathological fractures, and hypercalcemia. However, anti-resorptive therapies reduce the frequency of skeletal related events (such as episodes of b one pain and pathologic fractures) in women with breast cancer by only 50%. Additionally, one-third of patients develop further skeletal-related events within 2 years of initiating anti-resorptive therapy. These findings clearly indicate that other players, beyond the OC, modulate tumor bone metastases. By using immune competent mice we have recently demonstrated that reduced CD8+T cell responses can stimulate tumor growth in bone regardless of the OC status, thus reducing the efficiency of anti-resorptive ZOL treatment. This finding has highly clinical relevance since most patients with bone metastases are immune suppressed. Thus, we now aim to investigate the contribution of immune cells to tumor growth in bone. We are also interested in evaluating the beneficial therapeutic effects of immune therapies in combination with anti-resorptive treatments using animal models with established metastases to bones. The results from these proposed studies will be highly significant since have the potential to change the current therapeutic approaches used to treat patients with bone metastases.
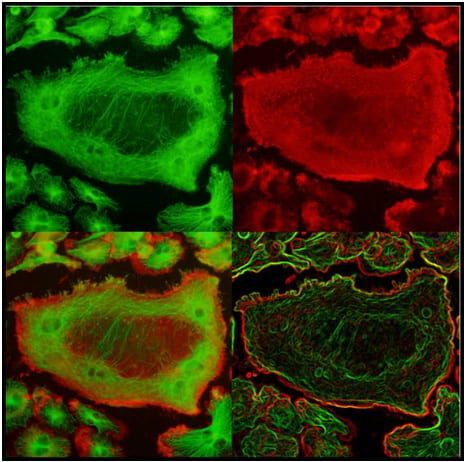
The lysosomal secretory apparatus represents an evolutionarily conserved mechanism that has been co-opted by many disparate cell types for specialized functions. Among cells that use secretory lysosomes, osteoclasts are unique in their remarkable capacity to secrete proteases and hydrolases that degrade bone tissue, a process required for the maintenance of optimal bone mass. To date, secretory lysosomes are known to be targeted to the membrane through vesicular trafficking molecules such as Rab7, Rab3 or SytVII in several cell types. This has been interpreted as evidence for a single secretory pathway for lysosomal vesicle secretion. We now find that the DAG/PKC pathway controls translocation of a specific pool of lysosomal vesicles, Cathepsin K but not v-ATPase vesicles. Importantly, DAG/ PKC pathway impairs bone resorption during basal bone homeostasis and protects from estrogen deficiency- induced bone loss. Thus, we are now seeking the mechanism by which PKC proteins control the exocytosis of a discrete pool of vesicles. We are also interested in investigating whether other hematopoietic cells containing secretory lysosomes, such as T cells, neutrophils and mast cells, utilize similar mechanisms to specifically mobilize discrete pools of lysosomes for exocytosis. Such flexibility would allow cells to secrete specialized lysosomal cargo in an appropriate context-specific manner.